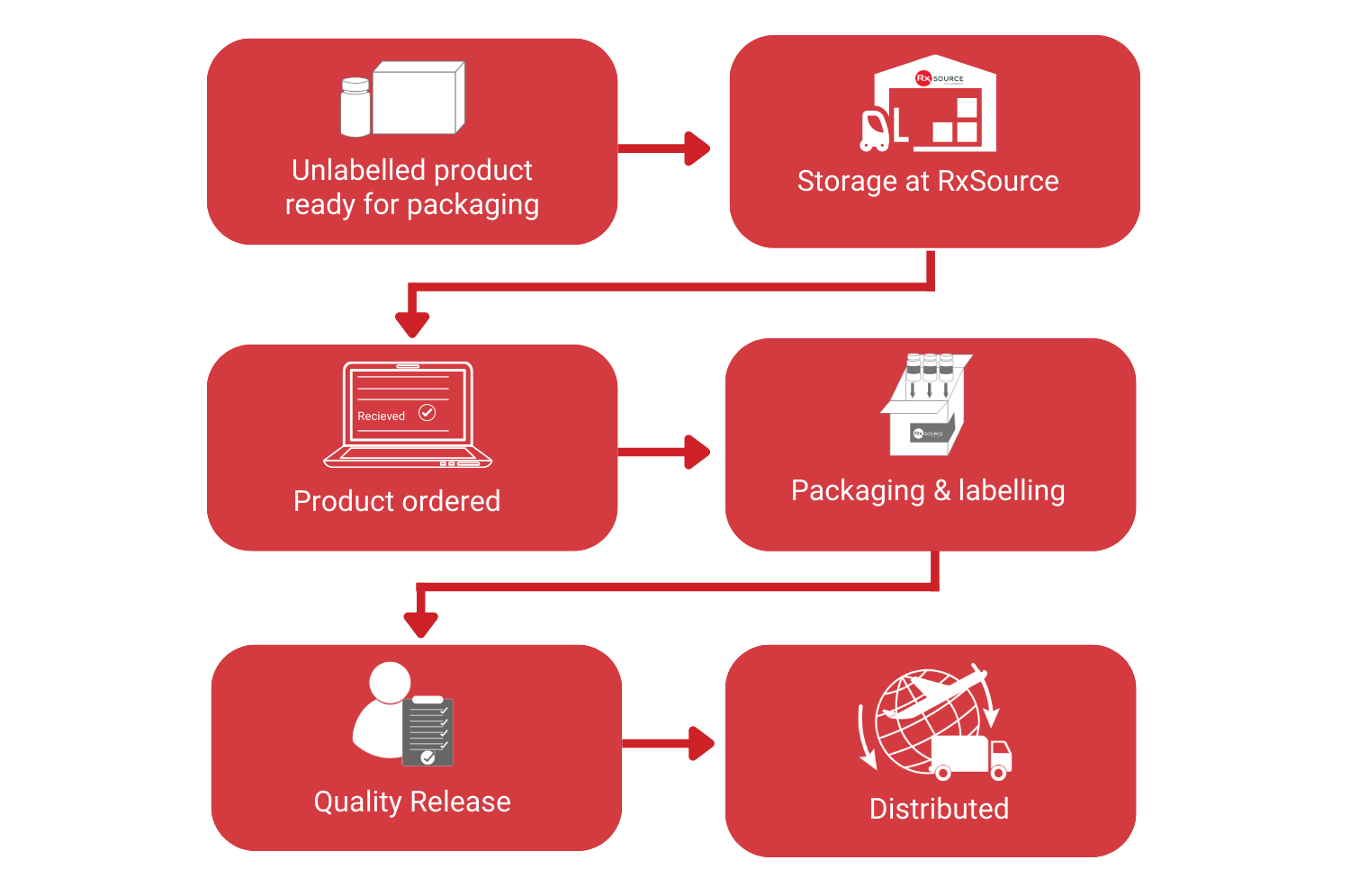
RxSource package kits to specific patient requirements in as little as 48 hours from the receipt of order.
By packaging on receipt of order, the best possible expiration date can be applied to each kit, reducing the need for future shelf-life extension labelling.
By not packaging until there is demand, we can store comparator products in their original commercial package. As it has not been manipulated, RxSource can help resell any material that isn’t used.
Last quarter alone, we were able to positively impact the lives of 44,500 patients. Our goal is to improve the lives of 10 million patients in 10 years. Contact us today to learn more about how we can work together to make a meaningful impact.
Contact Us